




Spatial regulation.pdf
免费下载

Spatial regulation and surface chemistry control of
monocyte/macrophage adhesion and foreign body giant
cell formation by photochemically micropatterned surfaces
Kristin M. DeFife,
1
Erica Colton,
1
Yasuhide Nakayama,
2
Takehisa Matsuda,
2
James M. Anderson
1
1
Institute of Pathology, Case Western Reserve University, Cleveland, Ohio 44125
2
Department of Bioengineering, National Cardiovascular Center Research Institute, Osaka 565, Japan
Received 14 August 1998; accepted 3 November 1998
Abstract: A long-standing goal of biomedical device devel-
opment has been the generation of specific, desired host
blood and tissue responses. An approach to meeting this
design criteria is precise surface modification that creates
micropatterns of distinct physicochemical character to direct
cell adhesion and behavior. For this study, poly(ethylene
terephthalate) films were coated with poly(benzyl N,N-
diethyldithiocarbamate-co-styrene) and sequentially ex-
posed to monomer solutions for photoirradiation. A photo-
mask was placed over different regions to generate micro-
patterned surfaces with graft polymer stripes of three
distinct ionic characters. Human monocytes were cultured
on these surfaces to ascertain whether adhesion and fusion
of monocytes/macrophages could be controlled. Nonionic
polyacrylamide greatly inhibited adhesion and induced
clumping of the few monocytes that did adhere. Macro-
phage adhesion and spreading led to high degrees of inter-
leukin-13 induced foreign body giant cell formation on both
the anionic poly(acrylic acid), sodium salt, and benzyl N,N-
diethyldithiocarbamate portions of the culture surface. In
spite of the highest observed levels of monocyte/macro-
phage adhesion on cationic poly(dimethylaminopropyl-
acrylamide), methiodide, the adherent cells were not com-
petent to undergo fusion to form foreign body giant cells.
These results suggest that inflammatory cell responses may
be spatially controlled in a manner that may be ultimately
exploited to improve the biocompatibility of medical de-
vices. © 1999 John Wiley & Sons, Inc. J Biomed Mater Res,
45, 148–154, 1999.
Key words: macrophage; foreign body giant cell; photo-
chemical micropattern; adhesion; interleukin-13
INTRODUCTION
The surface properties of functional biomedical de-
vices, such as advanced tissue-engineered materials
and microbiosensors, must control critical host re-
sponses during the foreign body reaction to implan-
tation.
1
Dimensionally precise surface control may be
employed to modulate regions of cellular behaviors,
such as adhesion, migration, proliferation, and activa-
tion, to create a desired pattern of response. Such sur-
face micropatterning mimics spatial control of cell be-
havior during tissue and organ development when
cell adhesion, motility, and activation are strictly con-
trolled.
2–4
To this end, photochemical techniques have been
developed to microprocess polymer surfaces to con-
tain clearly defined regions of chemically distinct
polymer.
5–7
Hydrophilic regions on hydrophobic sur-
faces and vice versa can be patterned with precision
on the order of microns via immobilization of benzyl
N,N-diethyldithiocarbamate onto a polymer and graft
copolymerization of monomers using a photomask
and UV irradiation.
5,6
Adhesion and orientation of a
variety of cell types, such as endothelial cells,
5–8
neural
cells,
9
and platelets,
10,11
have been controlled by these
methods. In general, low levels of cell adhesion oc-
curred on nonionic and hydrophilic (or very hydro-
phobic) surfaces. Cells preferentially adhered to mod-
erately hydrophobic or ionic surfaces.
An additional important biological design criteria is
the ability of these surfaces to affect the foreign body
reaction to implanted materials.
1
The inflammatory
and wound healing responses to implanted materials
are controlled by the extensive adhesive and secreto-
Correspondence to: Dr. J. M. Anderson
Contract grant sponsor: National Heart, Lung, and Blood
Institute, Devices and Technology Branch; Contract grant
number: HL 55714
Contract grant sponsor: The Whitaker Foundation
Contract grant sponsor: The Center for Cardiovascular
Biomaterials at Case Western Reserve University
Contract grant sponsor: Organization for Pharmaceutical
Safety and Research; Contract grant number: 97-15
© 1999 John Wiley & Sons, Inc. CCC 0021-9304/99/020148-07
ry capabilities of the monocyte-derived macro-
phage.
1,12–14
Moreover, persistent presence of the for-
eign material may support the cytokine-induced fu-
sion of macrophages to form multinucleated foreign
body giant cells (FBGC),
15–17
which can result in both
structural and functional failure of the implant. The
reactivity and versatility of inflammatory macro-
phages may impact the ability of a micropatterned
surface to control macrophage behavior in the same
manner as other cell types. Importantly, it is not
known if microprocessed copolymers can control det-
rimental FBGC formation.
In this study we utilized a hydrophobic polymer
surface that had three photograft copolymerized hy-
drophilic regions of distinct ionic character in conjunc-
tion with an in vitro cytokine-induced FBGC formation
protocol. The goals of this study were to elucidate the
effect of the micropatterned copolymers on critical
components of the development of the foreign body
reaction: human monocyte/macrophage adhesion, ad-
herent cell spreading, and macrophage fusion to form
FBGC.
MATERIALS AND METHODS
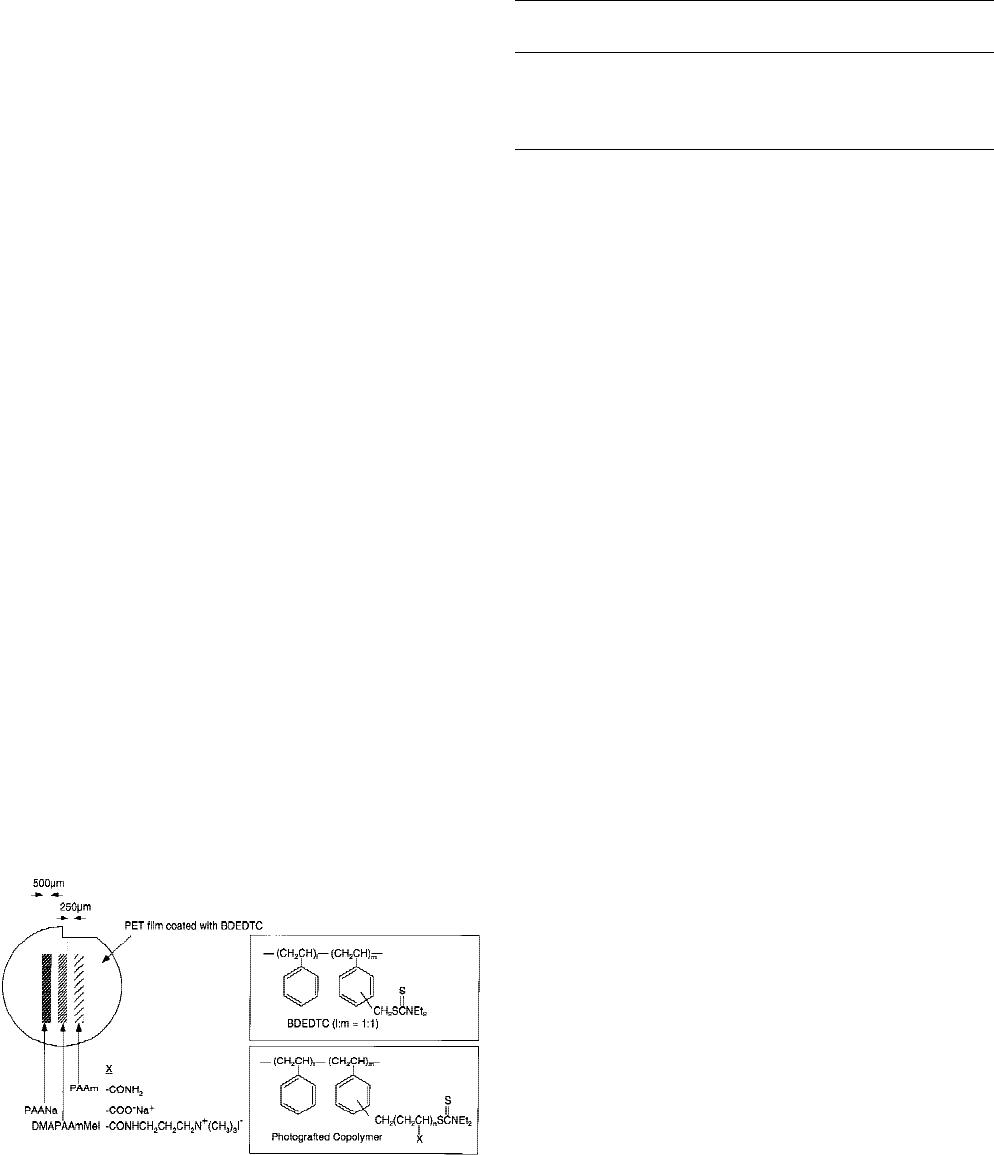
Culture surface preparation
Graft-polymerized samples were prepared with a custom-
designed, semiautomatic apparatus for laboratory-scale
mass production as described elsewhere.
5,18
Poly(ethylene
terephthalate) (PET) films were coated with poly(benzyl
N,N-diethyldithiocarbamate-co-styrene) (BDEDTC). Poly-
acrylamide (PAAm); sodium salt of poly(acrylic acid)
(PAANa); and methiodide of poly(dimethylaminopropyl-
acrylamide), (DMAPAAmMeI) were then photograft copo-
lymerized to the BDEDTC surface in an orientation shown
schematically in Figure 1. After sequential graft polymeriza-
tion, samples were cut into circles with a carbon dioxide
laser cutter. The advancing water contact angles were mea-
sured for each surface (Table I).
At least 1 day before the monocytes were to be cultured,
sample disks were immersed briefly in ethanol and placed
into sterile 24-well tissue culture polystyrene plates. Auto-
clave-sterilized silicone rings were used to secure the disks
in the bottom of the wells. Plates were wrapped in alumi-
num foil and stored in a sterile hood until use. Sample wells
were rinsed twice with sterile Dulbecco’s phosphate-
buffered saline (PBS; GIBCO, Grand Island, NY) before the
monocytes were added to the wells.
Monocyte isolation and culture
Human blood monocytes were isolated from the venous
blood of unmedicated donors by a nonadherent, density
centrifugation method.
19
Isolated monocytes were judged
>97% viable by Trypan Blue exclusion and >80% pure by
staining for nonspecific esterase and peroxidase. Monocytes
were suspended in a medium of RPMI-1640 (GIBCO) con-
taining 25% autologous serum and an antibiotic and anti-
mycotic mixture (GIBCO). Five × 10
5
monocytes in 0.5 mL of
medium were added to each sample well and were allowed
to adhere for2hat37°C in a humidified atmosphere of 95%
air and 5% CO
2
. Nonadherent cells were removed by aspi-
rating the medium and rinsing the wells with warmed
(37°C) PBS, and the remaining adherent monocytes were
covered with 1 mL per well of fresh medium. Cell results
termed day 0 were collected after this initial 2-h incubation.
On days 3 and 7 of incubation, the medium was replaced
with 25% heat-treated (56°C water bath for 1 h) autologous
serum in RPMI, and 10 ng/mL interleukin (IL)-13 (R & D
Systems, Minneapolis, MN) was added as indicated.
Samples were collected on days 0, 3, 7, and 10 by rinsing
the cultures twice with warmed (37°C) PBS and fixing for 5
min with methanol. Samples were stained with May–
Gru¨nwald/Giemsa as previously described
19
for light mi-
croscopic observation.
Evaluation of cell adhesion and FBGC formation
Cell adhesion was manually counted from three 40× ob-
jective fields for each condition, and results are expressed as
a percentage of the initial number of cultured cells (5 × 10
5
)
± the standard error of the mean (SEM, n = 3). Percent fusion
Figure 1. Photochemically microprocessed culture surface.
PET films were coated with poly(benzyl N,N-diethyldithio-
carbamate-co-styrene) (BDEDTC) and then photograft copo-
lymerized with polyacrylamide (PAAm); sodium salt of
poly(acrylic acid) (PAANa); and methiodide of poly(dimeth-
ylaminopropylacrylamide) (DMAPAAmMeI).
TABLE I
Evaluation of Photograft Copolymerized Polymer
Surface Chemistry
Polymer
Water Contact
Angle (°) Ionic Character
BDEDTC 83.4 ± 1.3 Nonionic
PAAm 31.6 ± 3.6 Nonionic
DMAPAAmMeI 29.2 ± 2.8 Cationic
PAANa 25.3 ± 3.3 Anionic
149MICROPATTERNED SURFACE CONTROL OF MC/M ADHESION AND FBGC FORMATION
was determined as the number of nuclei in the FBGCs (cells
with >2 nuclei) divided by the number of nuclei contained in
all adherent cells counted.
16
Nuclei were counted in three
20× objective fields for each condition, and results are ex-
pressed as percent fusion ± SEM (n = 3). The total area of cell
surface coverage was measured from three 20× objective
fields for each culture condition using the morphometric
software SigmaScan Pro (Jandel Scientific Software, San Ra-
fael, CA). Surface coverage was determined as the area cov-
ered by adherent cells divided by the total surface area and
is expressed as the percent surface coverage ± SEM (n = 3).
The unpaired Student’s t test was used for all statistical
analyses (StatView, Abacus Concepts, Inc., Berkeley, CA).
RESULTS
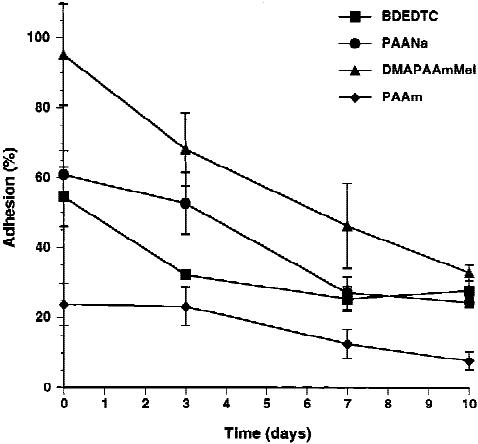
Monocyte/macrophage adhesion is modulated by
surface micropatterning
Human monocytes were cultured on BDEDTC-
coated PET with photochemically linked micropat-
terns of distinct ionic character (Fig. 1, Table I) to ask
whether surface-dependent patterning of monocyte/
macrophage adhesion could be achieved. Initial
monocyte adhesion was significantly affected by the
chemistry of the micropatterned surface (Fig. 2).
DMAPAAmMeI supported the highest degree of
monocyte adhesion after2hofculture, and adhesion
to PAAm was significantly lower than to any of the
other surfaces (p < 0.05). A culture time-dependent
decrease in adhesion was observed on all surfaces (p <
0.05) except for PAAm, which had a low degree of
initial adhesion. In general, monocyte/macrophage
adhesion to BDEDTC, PAANa, and DMAPAAmMeI
was not statistically different.
It was surprising that DMAPAAmMeI consistently
supported the most cell adhesion because it qualita-
tively appeared to support less adhesion than PAANa
and BDEDTC (Fig. 3). Monocytes/macrophages on
PAANa and BDEDTC appeared denser than those on
DMAPAAmMeI. In order to address this question, the
total surface area covered by the adherent cells was
measured. As shown in Figure 4, cell surface coverage
on PAANa and BDEDTC increased significantly with
culture time (p < 0.05) whereas surface coverage on
DMAPAAmMeI remained fairly constant for the en-
tire culture period. Surface coverage on PAAm was
always significantly lower than other surfaces, which
is consistent with the low degree of cell adhesion
(p < 0.05). By day 3 of culture, the monocytes/macro-
phages on PAANa were spreading, but on BDEDTC
the adherent cells were not contributing to an increase
in surface coverage, indicating that monocyte to mac-
rophage phenotypic development was delayed on this
surface compared to PAANa. By day 7 of culture, sur-
face coverage on BDEDTC and PAANa was equiva-
lent.
When surface coverage was expressed as a function
of cell adhesion, very different trends became appar-
ent among the micropatterned surfaces (Fig. 5). Very
few cells adhered to PAAm, and those that were pres-
ent clumped together and did not spread (Fig. 3). This
resulted in a very tight line on the plot in which no
points were statistically different. DMAPAAmMeI
provided the most linear trend (Fig. 5). The decrease in
cell number was fairly linear (Fig. 2), and the surface
coverage was fairly consistent (Fig. 4). The cells pro-
gressed to a macrophage morphology as judged by an
increase in size; however, these monocytes/macro-
phages became spindly and elongated (Fig. 3). Mono-
cytes on PAANa matured into macrophages, and ad-
herent cells spread to cover almost 90% of the avail-
able surface area; so even with fewer adherent cells
than DMAPAAmMeI, the surface coverage was
greater (Fig. 5). The trend on BDEDTC is similar to
PAANa and is not shown for clarity.
Macrophage competency to participate in FBGC
formation is controlled by surface micropatterns
IL-13 was added to the monocyte/macrophage cul-
tures to determine the effect of micropatterned sur-
faces on macrophage fusion to form FBGCs. Too few
cells adhered to PAAm to support macrophage fusion,
Figure 2. Micropattern-dependent monocyte/macrophage
adhesion. Human monocytes were cultured on BDEDTC-
coated PET films with three polymeric micropatterns of dis-
tinct ionic character. On the indicated days, cultures were
fixed and stained with May–Gru¨nwald/Giemsa to count the
numbers of adherent monocytes/macrophages. Percent ad-
hesion is expressed as mean ± SEM.
150 D
EFIFE ET AL.
of 7
免费下载
【版权声明】本文为墨天轮用户原创内容,转载时必须标注文档的来源(墨天轮),文档链接,文档作者等基本信息,否则作者和墨天轮有权追究责任。如果您发现墨天轮中有涉嫌抄袭或者侵权的内容,欢迎发送邮件至:contact@modb.pro进行举报,并提供相关证据,一经查实,墨天轮将立刻删除相关内容。
墨天轮 PM
最新上传
下载排行榜
1
2
9-数据库人的进阶之路:从PG分区、SQL优化到拥抱AI未来(罗敏).pptx
3
1-PG版本兼容性案例(彭冲).pptx
4
2-TDSQL PG在复杂查询场景中的挑战与实践-opensource.pdf
5
6-PostgreSQL 哈希索引原理浅析(文一).pdf
6
3-AI时代的变革者-面向机器的接口语言(MOQL)_吕海波.pptx
7
8-基于PG向量和RAG技术的开源知识库问答系统MaxKB.pptx
8
4-IvorySQL V4:双解析器架构下的兼容性创新实践.pptx
9
7-拉起PG好伙伴DifySupaOdoo.pdf
10
《云原生安全攻防启示录》李帅臻.pdf


相关文档
评论